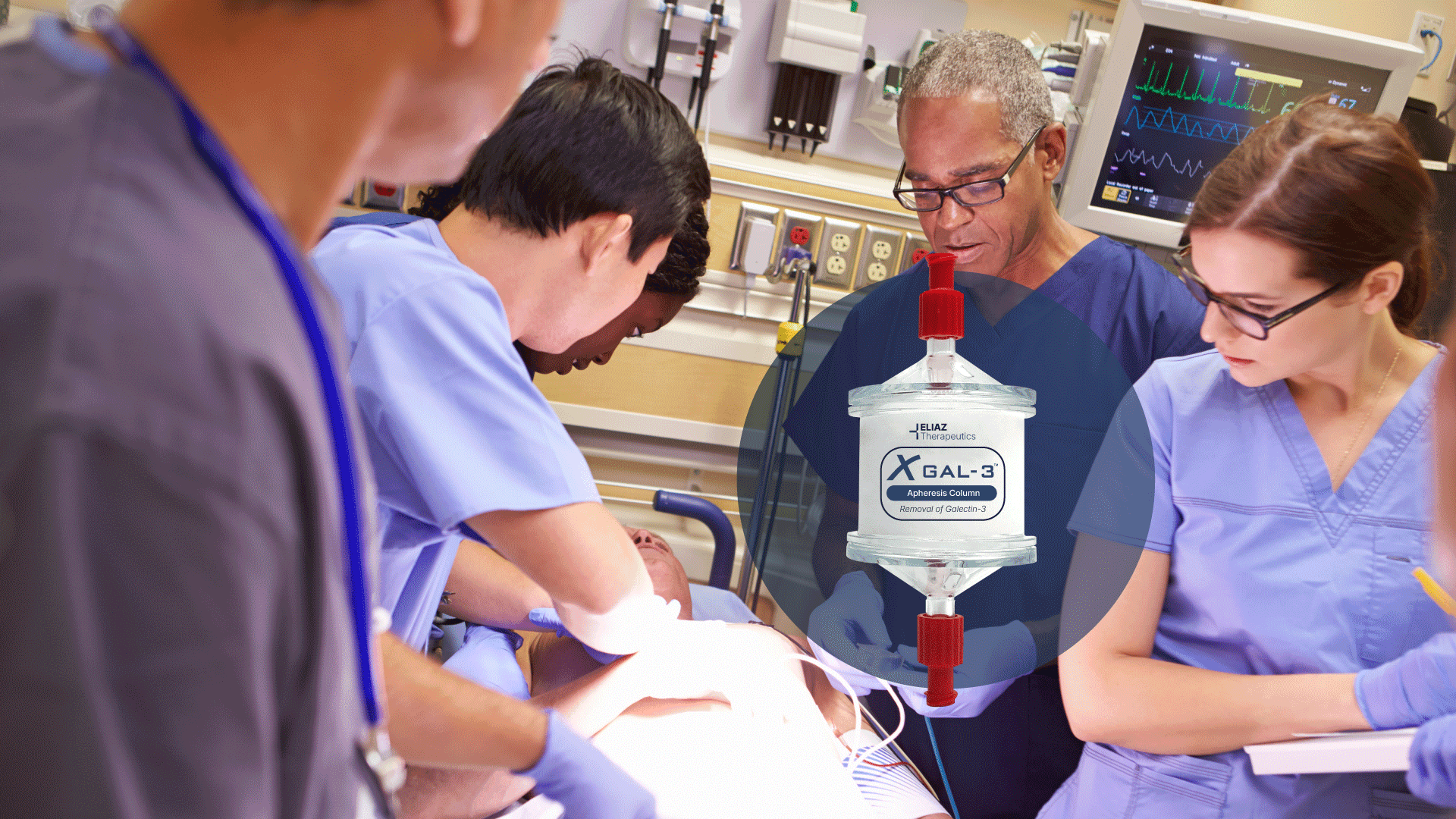
Every 2.8 seconds, someone dies from sepsis.
XGal-3® is the first device designed to remove the driver behind it.
The FDA-Recognized Technology to Fight Sepsis at the Source.
Our mission is to move beyond symptom management and treat sepsis at its source.
XGal-3® is an FDA Breakthrough-Designated apheresis column designed to remove Galectin-3—a protein shown to drive the deadly inflammatory response seen in sepsis and other life-threatening conditions.
With 60+ patents, and global manufacturing partnerships, we’re building the next standard in critical care intervention—starting with sepsis.
Why Removing Galectin-3 Matters:

FDA Breakthrough Status for addressing an urgent unmet need
Backed by successful animal proof-of-concept studies

Long term collaboration with Terumo BCT
Built to scale across ICU protocols and global hospital systems
A Platform Technology Powering Breakthroughs in Kidney Disease, Cancer Immunotherapy, and Beyond
Backed by the FDA. Funded by the NIH. Protected by Global IP.
XGal-3® has been recognized by leading institutions for its potential to change how we treat life-threatening conditions like sepsis. This recognition accelerates development, builds investor confidence, and clears a faster path to patients.
$1.9 Million in NIH Funding
Awarded for large-animal safety studies and additional R&D support.
60+ Patents Secured Across 34 Countries
Global protection for our platform technology and therapeutic method.
FDA Breakthrough Device Designation
Awarded to innovations expected to offer more effective treatment for serious or life-threatening diseases.
"Once I understood the role Galectin-3 plays in organ failure, sepsis, and preventable deaths—it was clear. This isn’t just a medical breakthrough. It’s a way to stop massive, avoidable suffering in hospitals everywhere. That’s why I invested."
Russell L. Brand
Investor & Advocate for Medical Innovation